Nine over-the-counter drug retailers, including CVS, Stop & Shop and Giant, are recalling two flavors of children’s liquid cold medicine products from their shelves because of a potential overdose risk.
The products are sold by distributors nationwide under a variety of brand names. They are being pulled because the dose cups have incorrect markings. Using the flawed cups has the potential to lead to overdosing, the manufacturer, Perrigo Company, told CBS News.
Perrigo released a statement about the recall in partnership with the U.S. Food and Drug Administration today.
The recall includes two batches of children’s guaifenesin grape liquid (100mg/5 mL) and three batches of children’s guaifenesin DM cherry liquid (100mg guaifenesin and 5mg dextromethorphan HBr/ 5 ml) sold in 4 oz. bottles with the dosage cup in a box.
The grape flavored products affected by the recall were sold under the H.E.B. label with lot number 5LK0592, and the CVS label with lot number 5MK0340, both with expiration dates of 08/2017.
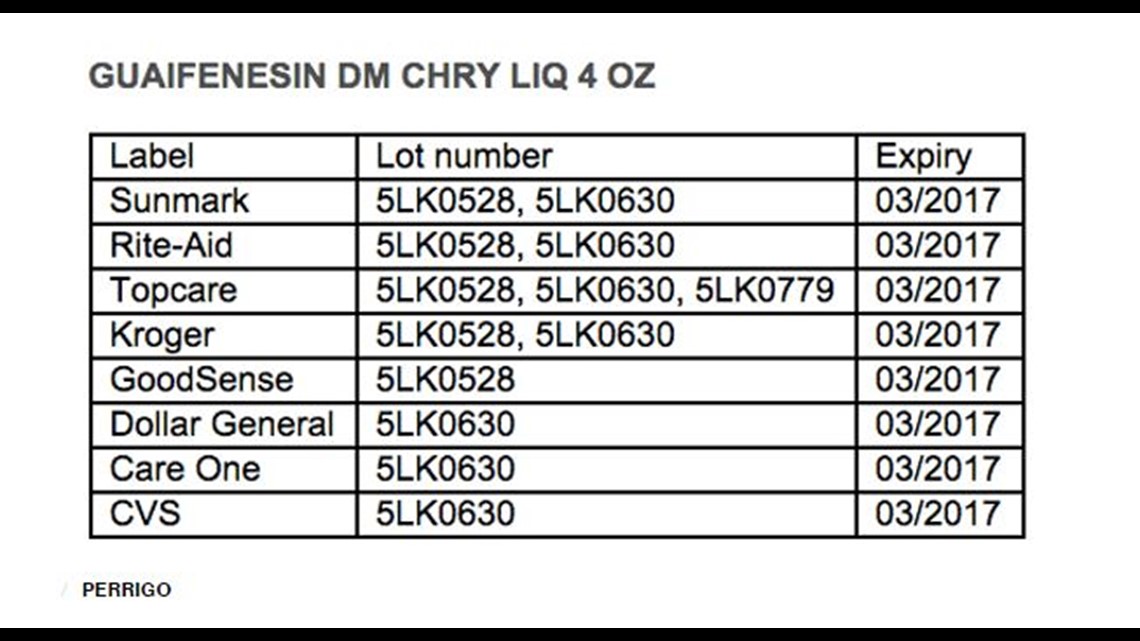
The store brand names and lot numbers for the cherry flavored products are listed in the chart below:

Stop & Shop and Giant were the first to announce a recall on Friday for all products with the UPC code 34152031837. Products with an expiration date of March 2017 are impacted, however all date codes were being pulled from shelves “out of an abundance of caution,” a recall announcement on Stop & Shop’s website said. Stop & Shop customer’s can return the recalled cough syrup for a full refund, and for more information, can call 1-800-767-7772.
An overdose of Guaifenesin DM may cause hyper excitability, rapid eye movements, changes in muscle reflexes, ataxia, dystonia, hallucinations, stupor, and coma, the Perrigo statement said. It said nausea, vomiting, tachycardia, irregular heartbeat, seizures, respiratory depression, and death can also occur with overdose.
The company said using the mislabeled cups is unlikely to result in serious side effects, and no reports related to overdose have been received to date.
However, taking the syrup regularly over several days at the incorrect dose could have a cumulative effect. Those most at risk include small children and anyone else who is a poor metabolizer of the drug dextromethorphan.
Perrigo’s consumer line is: 1-888-345-0479.



